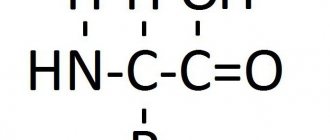Proteins, or proteins, are complex, high-molecular organic compounds consisting of amino acids. They represent the main, most important part of all cells and tissues of animal and plant organisms, without which vital physiological processes cannot take place. Proteins are different in their composition and properties in different animal and plant organisms and in different cells and tissues of the same organism. Proteins of different molecular compositions dissolve differently in water and in aqueous salt solutions; they do not dissolve in organic solvents. Due to the presence of acidic and basic groups in the protein molecule, it has a neutral reaction.
Proteins form numerous compounds with any chemical substances, which determines their special importance in chemical reactions occurring in the body and representing the basis of all manifestations of life and its protection from harmful influences. Proteins form the basis of enzymes, antibodies, hemoglobin, myoglobin, many hormones, and form complex complexes with vitamins.
By combining with fats and carbohydrates, proteins can be converted into fats and carbohydrates in the body during their breakdown. In the animal body, they are synthesized only from amino acids and their complexes - polypeptides, and they cannot be formed from inorganic compounds, fats and carbohydrates. Many low-molecular biologically active protein substances similar to those found in the body, such as some hormones, are synthesized outside the body.
General information about proteins and their classification
Proteins are the most important bioorganic compounds, which, along with nucleic acids, occupy a special role in living matter - without these compounds life is impossible, since, according to the definition of F. Engels, life is the special existence of protein bodies, etc.
“Proteins are natural biopolymers that are products of the polycondensation reaction of natural alpha amino acids.”
There are 18-23 natural alpha amino acids, their combination forms an infinitely large number of varieties of protein molecules, providing a variety of different organisms. Even individual organisms of a given species are characterized by their own proteins, and a number of proteins are found in many organisms.
Proteins are characterized by the following elemental composition: they are formed by carbon, hydrogen, oxygen, nitrogen, sulfur and some other chemical elements. The main feature of protein molecules is the obligatory presence of nitrogen atoms in them (in addition to the C, H, O atoms).
In protein molecules, a “peptide” bond is realized, that is, a bond between the C atom of the carbonyl group and the nitrogen atom of the amino group, which determines some of the features of protein molecules. The side chains of a protein molecule contain a large number of radicals and functional groups, which “makes” the protein molecule polyfunctional, capable of a significant variety of physicochemical and biochemical properties.
Due to the wide variety of protein molecules and the complexity of their composition and properties, proteins have several different classifications based on different characteristics. Let's look at some of them.
I. Based on their composition, two groups of proteins are distinguished:
1. Proteins (simple proteins; their molecule is formed only by protein, for example egg albumin).
2. Proteids are complex proteins whose molecules consist of protein and non-protein components.
Proteids are divided into several groups, the most important of which are:
1) glycoproteins (a complex combination of protein and carbohydrate);
2) lipoproteins (a complex of protein molecules and fats (lipids);
3) nucleoproteins (a complex of protein molecules and nucleic acid molecules).
II. Based on the shape of the molecule, two groups of proteins are distinguished:
1. Globular proteins - the protein molecule has a spherical shape (globule shape), for example, egg albumin molecules; such proteins are either soluble in water or capable of forming colloidal solutions.
2. Fibrillar proteins - the molecules of these substances have the form of threads (fibrils), for example, muscle myosin, silk fibroin. Fibrillar proteins are insoluble in water; they form structures that implement contractile, mechanical, shape-forming and protective functions, as well as the body’s ability to move in space.
III. Based on their solubility in various solvents, proteins are divided into several groups, of which the most important are the following:
1. Water soluble.
2. Fat soluble.
There are other classifications of proteins.
Brief characteristics of natural alpha amino acids
Natural alpha amino acids are a type of amino acid. An amino acid is a polyfunctional organic substance containing at least two functional groups - an amino group (-NH2) and a carboxyl (carboxylic, the latter is more correct) group (-COOH).
Alpha amino acids are amino acids in which the amino and carboxyl groups are located on the same carbon atom. Their general formula is NH2CH(R)COOH. Below are the formulas of some naturally occurring alpha amino acids; they are written in a form convenient for writing polycondensation reaction equations and are used when it is necessary to write reaction equations (schemes) for the production of certain polypeptides:
1) glycine (aminoacetic acid) - MH2CH2COOH;
2) alanine - NH2CH(CH3)COOH;
3) phenylalanine - NH2CH(CH2C6H5)COOH;
4) serine - NH2CH(CH2OH)COOH;
5) aspartic acid - NH2CH(CH2COOH)COOH;
6) cysteine - NH2CH(CH2SH)COOH, etc.
Some natural alpha amino acids contain two amino groups (for example, lysine), two carboxy groups (for example, aspartic and glutamic acids), hydroxide (OH) groups (for example, tyrosine), and can be cyclic (for example, proline).
Based on the nature of the influence of natural alpha amino acids on metabolism, they are divided into replaceable and irreplaceable. Essential amino acids must be supplied to the body through food.
Brief description of the structure of protein molecules
In addition to their complex composition, proteins are also characterized by the complex structure of protein molecules. There are four types of structures of protein molecules.
1. The primary structure is characterized by the order of arrangement of alpha amino acid residues in the polypeptide chain. For example, the tetrapeptide (a polypeptide formed by the polycondensation of four amino acid molecules) ala-phen-tyro-serine is a sequence of alanine, phenylalanine, tyrosine and serine residues linked to each other by a peptide bond.
2. The secondary structure of a protein molecule is the spatial arrangement of the polypeptide chain. It can be different, but the most common is the alpha helix, characterized by a certain “pitch” of the helix, the size and distance between the individual turns of the helix.
The stability of the secondary structure of the protein molecule is ensured by the emergence of various chemical bonds between the individual turns of the helix. The most important role among them belongs to the hydrogen bond (realized due to the retraction of the nucleus of the hydrogen atom of the groups - NH2 or =NH into the electron shell of oxygen or nitrogen atoms), ionic bond (realized due to the electrostatic interaction of the -COO- and - NH+3 or =NH ions +2) and other types of communication.
3. The tertiary structure of protein molecules is characterized by the spatial arrangement of the alpha helix or other structure. The stability of such structures is determined by the same types of connections as the secondary structure. As a result of the implementation of the tertiary structure, a “subunit” of the protein molecule arises, which is typical for very complex molecules, and for relatively simple molecules the tertiary structure is final.
4. The quaternary structure of a protein molecule is the spatial arrangement of the subunits of protein molecules. It is characteristic of complex proteins, such as hemoglobin.
When considering the structure of protein molecules, it is necessary to distinguish between the structure of a living protein - the native structure and the structure of a dead protein. Protein in living matter (native protein) is different from protein that has been subjected to a change in which it may lose the properties of a living protein. Shallow exposure is called denaturation, during which the properties of the living protein can subsequently be restored. One type of denaturation is reversible coagulation. With irreversible coagulation, the native protein turns into “dead protein.”
Brief description of the physical, physicochemical and chemical properties of protein
The properties of protein molecules are of great importance for the realization of their biological and environmental properties. Thus, according to their state of aggregation, proteins are classified as solid substances that can be soluble or insoluble in water or other solvents. Much of the bioecological role of proteins is determined by physical properties. Thus, the ability of protein molecules to form colloidal systems determines their construction, catalytic and other functions. The insolubility of proteins in water and other solvents, their fibrillarity determines the protective and shape-forming functions, etc.
The physicochemical properties of proteins include their ability to denature and coagulate. Coagulation manifests itself in colloidal systems, which are the basis of any living substance. During coagulation, particles become larger due to their sticking together. Coagulation can be hidden (it can only be observed under a microscope) and obvious - its sign is the precipitation of protein. Coagulation is irreversible when, after the cessation of the action of the coagulating factor, the structure of the colloidal system is not restored, and reversible when, after removal of the coagulating factor, the colloidal system is restored.
An example of reversible coagulation is the precipitation of egg albumin protein under the influence of salt solutions, while the protein precipitate dissolves when the solution is diluted or when the precipitate is transferred to distilled water.
An example of irreversible coagulation is the destruction of the colloidal structure of the protein albumin when heated to the boiling point of water. During death (complete), living matter turns into dead matter due to irreversible coagulation of the entire system.
The chemical properties of proteins are very diverse due to the presence of a large number of functional groups in protein molecules, as well as due to the presence of peptide and other bonds in protein molecules. From an ecological and biological point of view, the greatest importance is the ability of protein molecules to hydrolyze (this ultimately results in a mixture of natural alpha amino acids that participated in the formation of this molecule; there may be other substances in this mixture if the protein was a protein), to oxidation (its products can be carbon dioxide, water, nitrogen compounds, for example, urea, phosphorus compounds, etc.).
Proteins burn with the release of the smell of “burnt horn” or “burnt feathers,” which is necessary to know when conducting simple environmental experiments. Various color reactions to protein are known (biuret, xanthoprotein, etc.); more details about them can be found in the chemistry course.
Brief description of the ecological and biological functions of proteins
It is necessary to distinguish between the ecological and biological role of proteins in cells and in the body as a whole.
Ecological and biological role of proteins in cells
Due to the fact that proteins (along with nucleic acids) are the substances of life, their functions in cells are very diverse.
1. The most important function of protein molecules is the structural function, which consists in the fact that protein is the most important component of all structures that form the cell, in which it is included as part of a complex of various chemical compounds.
2. Protein is the most important reagent in the course of a huge variety of biochemical reactions that ensure the normal functioning of living matter, therefore it is characterized by a reagent function.
3. In living matter, reactions are possible only in the presence of biological catalysts - enzymes, and as established as a result of biochemical studies, enzymes are of a protein nature, therefore proteins also perform a catalytic function.
4. If necessary, proteins are oxidized in organisms and energy is released, due to which ATP is synthesized, i.e. proteins also perform an energy function, but due to the fact that these substances have a special value for organisms (due to their complex composition), the energy function of proteins is realized by organisms only under critical conditions.
5. Proteins can also perform a storage function, since they are a kind of “canned food” of substances and energy for organisms (especially plants), ensuring their initial development (for animals - intrauterine, for plants - the development of embryos until the appearance of a young organism - a seedling).
A number of protein functions are characteristic of both cells and the body as a whole, therefore they are discussed below.
Ecological and biological role of proteins in organisms (in general)
1. Proteins form special structures in cells and organisms (in combination with other substances) that are capable of perceiving signals from the environment in the form of irritations, due to which a state of “excitation” arises, to which the body responds with a certain reaction, i.e. Proteins both in the cell and in the body as a whole are characterized by a perceptive function.
2. Proteins are also characterized by a conductive function (both in cells and in the body as a whole), which consists in the fact that the excitation that arises in certain structures of the cell (organism) is transmitted to the corresponding center (cell or organism), in which a certain reaction is formed ( response) of an organism or cell to a received signal.
3. Many organisms are capable of moving in space, which is possible due to the ability of the structures of the cell or organism to contract, and this is possible because proteins of the fibrillar structure have a contractile function.
4. For heterotrophic organisms, proteins, both separately and in mixture with other substances, are food products, that is, they are characterized by a trophic function.
Brief description of protein transformations in heterotrophic organisms using the example of humans
Proteins in food enter the oral cavity, where they are moistened with saliva, crushed by teeth and transformed into a homogeneous mass (with thorough chewing), and through the pharynx and esophagus enter the stomach (until they enter the latter, nothing happens to the proteins as compounds).
In the stomach, the food bolus is saturated with gastric juice, which is the secretion of the gastric glands. Gastric juice is an aqueous system containing hydrogen chloride and enzymes, the most important of which (for proteins) is pepsin. Pepsin in an acidic environment causes the hydrolysis of proteins to peptones. The food gruel then enters the first section of the small intestine - the duodenum, into which the pancreatic duct opens, secreting pancreatic juice, which has an alkaline environment and a complex of enzymes, of which trypsin accelerates the process of protein hydrolysis and leads it to the end, i.e. until the appearance of mixtures of natural alpha amino acids (they are soluble and can be absorbed into the blood by the intestinal villi).
This mixture of amino acids enters the interstitial fluid, and from there into the cells of the body, in which they (amino acids) enter into various transformations. One part of these compounds is directly used for the synthesis of proteins characteristic of a given organism, the second is subjected to transamination or deamination, giving new compounds necessary for the body, the third is oxidized and is a source of energy necessary for the body to carry out its vital functions.
It is necessary to note some features of intracellular protein transformations. If the organism is heterotrophic and unicellular, then the proteins in the food enter the cells into the cytoplasm or special digestive vacuoles, where they undergo hydrolysis under the action of enzymes, and then everything proceeds as described for amino acids in cells. Cellular structures are constantly renewed, so the “old” protein is replaced with a “new” one, while the first one is hydrolyzed to produce a mixture of amino acids.
Autotrophic organisms have their own characteristics in protein transformations. Primary proteins (in meristem cells) are synthesized from amino acids, which are synthesized from the products of transformations of primary carbohydrates (they arose during photosynthesis) and inorganic nitrogen-containing substances (nitrates or ammonium salts). The replacement of protein structures in long-living cells of autotrophic organisms does not differ from that for heterotrophic organisms.
Amino acid composition of proteins
Proteins are non-periodic polymers whose monomers are α-amino acids . Typically, 20 types of α-amino acids are called protein monomers, although over 170 of them are found in cells and tissues.
Depending on whether amino acids can be synthesized in the human body and other animals, they are distinguished: non-essential amino acids - can be synthesized; essential amino acids - cannot be synthesized. Essential amino acids must be supplied to the body through food. Plants synthesize all types of amino acids.
Depending on the amino acid composition, proteins are: complete - contain the entire set of amino acids; defective - some amino acids are missing in their composition. If proteins consist only of amino acids, they are called simple . If proteins contain, in addition to amino acids, a non-amino acid component (prosthetic group), they are called complex . The prosthetic group can be represented by metals (metalloproteins), carbohydrates (glycoproteins), lipids (lipoproteins), nucleic acids (nucleoproteins).
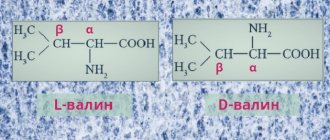
All amino acids contain : 1) a carboxyl group (–COOH), 2) an amino group (–NH2), 3) a radical or R-group (the rest of the molecule). The structure of the radical is different for different types of amino acids. Depending on the number of amino groups and carboxyl groups included in the composition of amino acids, they are distinguished: neutral amino acids , having one carboxyl group and one amino group; basic amino acids having more than one amino group; acidic amino acids having more than one carboxyl group.
Amino acids are amphoteric compounds , since in solution they can act as both acids and bases. In aqueous solutions, amino acids exist in different ionic forms.
Nitrogen balance
Proteins, made up of amino acids, are the basic compounds essential to the processes of life. Therefore, it is extremely important to take into account the metabolism of proteins and their breakdown products.
The nitrogen content in proteins averages 16% of their mass. Therefore, by determining the amount of nitrogen entering the body with food and the amount of nitrogen in urine, sweat and feces, it is possible to calculate the protein, or nitrogen, balance of the body.
There is very little nitrogen in sweat, so sweat analysis for nitrogen content is not usually done. The amount of nitrogen received from food and the amount of nitrogen contained in urine and feces are multiplied by 6.25 (16%) and the second is subtracted from the first value. As a result, the amount of nitrogen entered and absorbed by the body is determined.
When the amount of nitrogen entering the body with food is equal to the amount of nitrogen in the urine and feces, i.e., formed during deamination, then there is nitrogen equilibrium. Nitrogen balance is characteristic, as a rule, of a healthy adult organism.
When the amount of nitrogen entering the body is greater than the amount of nitrogen excreted, then there is a positive nitrogen balance, i.e., the amount of protein included in the body is greater than the amount of protein that has undergone decomposition. A positive nitrogen balance is characteristic of a growing healthy organism.
When dietary protein intake increases, the amount of nitrogen excreted in urine also increases.
And finally, when the amount of nitrogen entering the body is less than the amount of nitrogen excreted, then there is a negative nitrogen balance, in which the breakdown of protein exceeds its synthesis and the protein that makes up the body is destroyed. This happens during protein starvation and when the amino acids necessary for the body are not supplied. A negative nitrogen balance was also found after exposure to large doses of ionizing radiation, which cause increased breakdown of proteins in organs and tissues.
The protein optimum problem
The minimum amount of food proteins required to replenish the deteriorating proteins of the body, or the amount of breakdown of body proteins with an exclusively carbohydrate diet, is designated as the wear coefficient. In an adult, the smallest value of this coefficient is about 30 g of protein per day. However, this quantity is not enough.
Fats and carbohydrates influence the consumption of proteins beyond the minimum required for plastic purposes, since they release the amount of energy that was required for the breakdown of proteins above the minimum. Carbohydrates during normal nutrition reduce the breakdown of proteins by 3-3.5 times more than during complete starvation.
For an adult with mixed food containing a sufficient amount of carbohydrates and fats, and a body weight of 70 kg, the protein norm per day is 105 g.
The amount of protein that fully ensures the growth and vital activity of the body is designated as the protein optimum and is equal to 100-125 g of protein per day for a person during light work, up to 165 g per day during hard work, and 220-230 g during very hard work.
The amount of protein per day should be at least 17% of the total food by weight, and 14% by energy.
Review of popular protein diets
Protein diets are very popular and quite effective, since the main sources of food for this form of weight loss are dishes high in protein. Diets of this kind have many advantages and disadvantages.
Advantages:
- Protein is the best component for restoring energy if you combine diet with exercise;
- protein diets are designed for no more than 7 days, and this is a fairly quick achievement of results in a short period of time;
- Protein foods satiate the body for a long time, which means that this diet is not one of the “hungry” diets.
Minuses:
- with unlimited protein consumption, the human body removes fluid and calcium reserves;
- Also, playing sports provokes an increased load on the kidneys, which will affect the appearance: the skin dries, the hair loses its color saturation, the nails peel off;
- when on a protein diet, you need to alternate product categories to avoid allergies;
- You should carefully monitor the amount of fat.
The list of foods that you can eat on a protein diet is in the table:
Complete and incomplete proteins
Proteins that enter the body with food are divided into biologically complete and biologically incomplete.
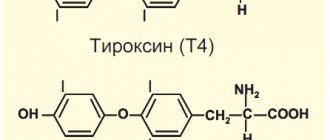
Biologically complete proteins are those that contain in sufficient quantities all the amino acids necessary for the synthesis of protein in an animal body. Complete proteins necessary for the growth of the body include the following essential amino acids: lysine, tryptophan, threonine, leucine, isoleucine, histidine, arginine, valine, methionine, phenylalanine. From these amino acids other amino acids, hormones, etc. can be formed. Tyrosine is formed from phenylalanine, the hormones thyroxine and adrenaline are formed from tyrosine through transformations, and histamine is formed from histidine. Methionine is involved in the formation of thyroid hormones and is necessary for the formation of choline, cysteine and glutathione. It is necessary for redox processes, nitrogen metabolism, fat absorption, and normal brain activity. Lysine is involved in hematopoiesis and promotes body growth. Tryptophan is also necessary for growth, participates in the formation of serotonin, vitamin PP, and tissue synthesis. Lysine, cystine and valine stimulate cardiac activity. A low content of cystine in food delays hair growth and increases blood sugar.
Biologically deficient proteins are those that lack even one amino acid that cannot be synthesized by animal organisms.
The biological value of protein is measured by the amount of body protein that is formed from 100 g of food protein.
Proteins of animal origin, found in meat, eggs and milk, are the most complete (70-95%). Proteins of plant origin have less biological value, for example proteins of rye bread, corn (60%), potatoes, yeast (67%).
Animal protein - gelatin, which does not contain tryptophan and tyrosine, is inferior. Wheat and barley are low in lysine, and corn is low in lysine and tryptophan.
Some amino acids replace each other, for example phenylalanine replaces tyrosine.
Two incomplete proteins, which lack various amino acids, together can form a complete protein diet.
The role of the liver in protein synthesis
The liver synthesizes proteins contained in blood plasma: albumins, globulins (with the exception of gamma globulins), fibrinogen, nucleic acids and numerous enzymes, some of which are synthesized only in the liver, for example enzymes involved in the formation of urea.
Proteins synthesized in the body are part of organs, tissues and cells, enzymes and hormones (the plastic meaning of proteins), but are not stored by the body in the form of various protein compounds. Therefore, that part of the proteins that does not have plastic significance is deaminated with the participation of enzymes - decomposes with the release of energy into various nitrogenous products. The half-life of liver proteins is 10 days.
Protein digestibility coefficient
As mentioned above, digestibility is one of the main indicators of protein quality, which can vary among different food groups. Therefore, when choosing foods that contain a lot of protein, you should also take into account information about how much protein will be absorbed.
The table below shows products with a protein content above average and their digestibility coefficients (the amount of protein absorbed by the body per 100 grams of product).
Summary table of protein content in food products and its digestibility coefficient
| Product name | Digestibility coefficient |
| Caviar | 24 |
| Turkey meat | 23 |
| Sardine | 22 |
| Tuna | 21 |
| Hard cheeses | 21 |
| Chum salmon | 20 |
| Chicken meat | 17 |
| Pork liver | 17 |
| Beef | 15 |
| Beans | 15 |
| Soybeans | 15 |
| Peas | 15 |
Protein nutrition under various conditions
Undigested protein cannot be absorbed by the body except through the digestive canal. Protein introduced outside the digestive canal (parenterally) causes a protective reaction from the body.
Amino acids of the broken down protein and their compounds - polypeptides - are brought by the blood to the cells of the body, in which, under the influence of enzymes, protein synthesis occurs continuously throughout life. Food proteins have mainly plastic significance.
During the period of growth of the body - in childhood and adolescence - protein synthesis is especially high. In old age, protein synthesis decreases. Consequently, during the growth process, retention or retention in the body of the chemical elements that make up proteins occurs.
The study of metabolism using isotopes has shown that in some organs, within 2-3 days, approximately half of all proteins undergo breakdown and the same amount of proteins is newly synthesized by the body (resynthesis). In each tissue, in each organism, specific proteins are synthesized that differ from the proteins of other tissues and other organisms.
Like fats and carbohydrates, amino acids that are not used to build the body are broken down to release energy.
Amino acids, which are formed from the proteins of dying, collapsing cells of the body, also undergo transformations with the release of energy.
Under normal conditions, the amount of protein required per day for an adult is 1.5-2.0 g per 1 kg of body weight, in conditions of prolonged cold 3.0-3.5 g, with very heavy physical work 3.0-3.5 G.
An increase in the amount of proteins to more than 3.0-3.5 g per 1 kg of body weight disrupts the activity of the nervous system, liver and kidneys.