Chemical substances containing the structural components of a carboxylic acid and an amine molecule are called amino acids. This is the general name for a group of organic compounds that contain a hydrocarbon chain, a carboxyl group (-COOH) and an amino group (-NH2). Their precursors are carboxylic acids, and molecules in which the hydrogen at the first carbon atom is replaced by an amino group are called alpha amino acids.
Only 20 amino acids are valuable for enzymatic biosynthesis reactions that occur in the body of all living beings. These substances are called standard amino acids. There are also non-standard amino acids that are included in some special protein molecules. They are not found everywhere, although they perform an important function in wildlife. It is likely that the radicals of these acids are modified after biosynthesis.
General information and list of substances
There are two large groups of amino acids that were isolated due to the patterns of their occurrence in nature. Specifically, there are 20 standard type amino acids and 26 non-standard type amino acids. The former are found in the proteins of any living organism, while the latter are specific to individual living organisms.
The 20 standard amino acids are divided into 2 types depending on their ability to be synthesized in the human body. These are replaceable, which in human cells can be formed from precursors, and irreplaceable, for the synthesis of which there are no enzyme systems or substrate. Nonessential amino acids may not be present in food, since the body can synthesize them, replenishing their quantity if necessary. Essential amino acids cannot be obtained by the body on its own and therefore must be obtained from food.
Biochemists have determined the names of amino acids from the group of essential amino acids. There are 8 known in total:
- methionine;
- threonine;
- isoleucine;
- leucine;
- phenylalanine;
- tryptophan;
- valine;
- lysine;
- histidine is also often included here.
These are substances with a different structure of the hydrocarbon radical, but always with the presence of a carboxyl group and an amino group at the alpha-C atom.
There are 11 substances in the group of non-essential amino acids:
- alanine;
- glycine;
- arginine;
- asparagine;
- aspartic acid;
- cysteine;
- glutamic acid;
- glutamine;
- proline;
- serine;
- tyrosine
Basically, their chemical structure is simpler than that of the essential ones, so their synthesis is easier for the body. Most essential amino acids cannot be obtained only due to the lack of a substrate, that is, a precursor molecule through a transamination reaction.
Glycine, alanine, valine
In the biosynthesis of protein molecules, glycine, valine and alanine are most often used (the formula of each substance is indicated below in the figure). These amino acids are the simplest in chemical structure. The substance glycine is the simplest in the class of amino acids, that is, in addition to the alpha carbon atom, the compound has no radicals. However, even the simplest molecule in structure plays an important role in ensuring life. In particular, the porphyrin ring of hemoglobin and purine bases are synthesized from glycine. The porphyry ring is a protein section of hemoglobin, designed to hold iron atoms as part of an integral substance.
Glycine is involved in the functioning of the brain, acting as an inhibitory transmitter of the central nervous system. This means that it is more involved in the work of the cerebral cortex - its most complexly organized tissue. More importantly, glycine is a substrate for the synthesis of purine bases necessary for the formation of nucleotides that encode hereditary information. In addition, glycine serves as a source for the synthesis of the other 20 amino acids, while it itself can be formed from serine.
The amino acid alanine has a slightly more complex formula than glycine, since it has a methyl radical replaced by one hydrogen atom at the alpha carbon atom of the substance. At the same time, alanine also remains one of the molecules most often involved in the processes of protein biosynthesis. It is part of any protein in living nature.
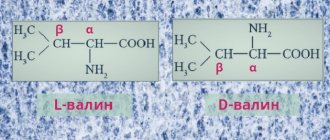
Valine, which cannot be synthesized in the human body, is an amino acid with a branched hydrocarbon chain consisting of three carbon atoms. The isopropyl radical gives the molecule more weight, but because of this it is impossible to find a substrate for biosynthesis in the cells of human organs. Therefore, valine must be supplied with food. It is present predominantly in the structural proteins of muscles.
Research results confirm that valine is essential for the functioning of the central nervous system. In particular, due to its ability to restore the myelin sheath of nerve fibers, it can be used as an adjuvant in the treatment of multiple sclerosis, drug addiction, and depression. It is found in large quantities in meat products, rice, and dried peas.
Leucine in sports: a bodybuilder's true love. BCAA dietary supplement
It must be said that leucine is the main component (more than 70%) of the popular BCAA dietary supplement. Today, leucine is increasingly used in sports. It's no secret that bodybuilders have a special love for it. But if an athlete takes only leucine, then he will need to eat it with spoons, and such volumes are harmful to the kidneys. In addition, complete absorption of amino acids occurs in the presence of B vitamins. It has also been found that two other branched chain amino acids, isoleucine and valine, greatly enhance the effect of leucine.
Interestingly, leucine, along with other BCAAs, causes the body to release serine and threonine, which form a phosphorylating current . This effect stimulates protein synthesis, which is necessary for muscle growth . That's why this amino acid is so loved by bodybuilders and weightlifters. And indeed, correctly, using its capabilities, you can accelerate the growth of muscle mass. The most commonly used ratio of leucine to isoleucine to valine by BCAA supplement companies is 2:1:1. Trainers recommend taking this sports nutrition before and immediately after training to take advantage of the protein-carbohydrate window. A special effect can be achieved by consuming the supplement during the exercise itself - every 20 minutes.
Tyrosine, histidine, tryptophan
In the body, tyrosine can be synthesized from phenylalanine, although it comes in large quantities from dairy foods, mainly cottage cheese and cheeses. It is part of casein, an animal protein found in abundance in curd and cheese products. The key significance of tyrosine is that its molecule becomes a substrate for the synthesis of catecholamines. These are adrenaline, norepinephrine, and dopamine - mediators of the humoral system for regulating body functions. Tyrosine is able to quickly penetrate the blood-brain barrier, where it quickly turns into dopamine. The tyrosine molecule is involved in melanin synthesis, providing pigmentation to the skin, hair and iris.
The amino acid histidine is part of the structural and enzymatic proteins of the body and is a substrate for the synthesis of histamine. The latter regulates gastric secretion, participates in immune reactions, and regulates the healing of damage. Histidine is an essential amino acid, and the body replenishes its reserves only from food.
Tryptophan is also unable to be synthesized by the body due to the complexity of its hydrocarbon chain. It is part of proteins and is a substrate for the synthesis of serotonin. The latter is a neurotransmitter designed to regulate the cycles of wakefulness and sleep. Tryptophan and tyrosine - these names of amino acids should be remembered by neurophysiologists, since they synthesize the main mediators of the limbic system (serotonin and dopamine), which ensure the presence of emotions. However, there is no molecular form that ensures the accumulation of essential amino acids in tissues, which is why they must be present in food daily. Protein food in the amount of 70 grams per day fully meets these needs of the body.
What foods contain the amino acid leucine?
The compound can only be taken into the body through food. A balanced diet with the inclusion of predominantly animal products in the diet is essential.
The main sources of the substance include:
- egg powder;
- soy protein concentrate;
- caviar;
- cheeses;
- seafood (squid);
- legumes (beans, peas);
- fish (perch, mackerel, pike perch, pike, herring);
- meat (turkey, beef, lamb, lean pork);
- nuts (peanuts, hazelnuts, almonds, sesame, pistachios);
- sunflower seeds;
- chicken egg;
- corn grits.
We recommend reading: What are the benefits of almonds, properties and contraindications
Minor amounts of the organic compound include mushrooms, vegetables and fruits.
Attention! Alcoholic drinks interfere with the absorption of essential amino acids.
Phenylalanine, leucine and isoleucine
Phenylalanine is notable for the fact that the amino acid tyrosine is synthesized from it when it is deficient. Phenylalanine itself is a structural component of all proteins in living nature. It is a metabolic precursor to the neurotransmitter phenylethylamine, providing mental focus, mood enhancement, and psychostimulation. In the Russian Federation, circulation of this substance in concentrations above 15% is prohibited. The effect of phenylethylamine is similar to that of amphetamine, but the former does not have a harmful effect on the body and differs only in the development of mental dependence.
One of the main substances of the amino acid group is leucine, from which the peptide chains of any human protein, including enzymes, are synthesized. The compound, used in its pure form, is capable of regulating liver functions, accelerating the regeneration of its cells, and ensuring rejuvenation of the body. Therefore, leucine is an amino acid that is available in the form of a drug. It is highly effective in the auxiliary treatment of liver cirrhosis, anemia, and leukemia. Leucine is an amino acid that significantly facilitates the rehabilitation of patients after chemotherapy.
Isoleucine, like leucine, is not able to be synthesized by the body independently and belongs to the group of essential ones. However, this substance is not a medicine, since the body has little need for it. Basically, only one stereoisomer (2S,3S)-2-amino-3-methylpentanoic acid is involved in biosynthesis.
Proline, serine, cysteine
The substance proline is an amino acid with a cyclic hydrocarbon radical. Its main value is the presence of a ketone group in the chain, which is why the substance is actively used in the synthesis of structural proteins. Reduction of the heterocycle ketone to a hydroxyl group to form hydroxyproline forms multiple hydrogen bonds between collagen chains. As a result, the threads of this protein intertwine and provide a strong intermolecular structure.
Proline is an amino acid that provides mechanical strength to human tissue and its skeleton. Most often it is found in collagen, which is part of bones, cartilage and connective tissue. Like proline, cysteine is an amino acid from which structural protein is synthesized. However, this is not collagen, but a group of alpha-keratin substances. They form the stratum corneum of the skin, nails, and are present in hair scales.
The substance serine is an amino acid that exists in the form of optical L and D isomers. This is a nonessential substance synthesized from phosphoglycerate. Serine can be formed during an enzymatic reaction from glycine. This interaction is reversible, and therefore glycine can be formed from serine. The main value of the latter is that enzymatic proteins, or rather their active centers, are synthesized from serine. Serine is widely present in structural proteins.
Arginine, methionine, threonine
Biochemists have determined that excessive consumption of arginine provokes the development of Alzheimer's disease. However, in addition to the negative meaning, the substance also has functions that are vital for reproduction. In particular, due to the presence of a guanidine group, which resides in the cell in cationic form, the compound is capable of forming a huge number of intermolecular hydrogen bonds. Thanks to this, arginine in the form of a zwitterion acquires the ability to bind to the phosphate regions of DNA molecules. The result of the interaction is the formation of many nucleoproteins - the packaging form of DNA. Arginine, when changing the pH of the nuclear matrix of the cell, can be detached from the nucleoprotein, providing unwinding of the DNA chain and the beginning of translation for protein biosynthesis.
The amino acid methionine contains a sulfur atom in its structure, which is why the pure substance in crystalline form has an unpleasant rotten odor due to the released hydrogen sulfide. In the human body, methionine performs a regenerative function, promoting the healing of liver cell membranes. Therefore, it is available in the form of an amino acid preparation. A second drug intended for diagnosing tumors is also synthesized from methionine. It is synthesized by replacing one carbon atom with its C11 isotope. In this form, it actively accumulates in tumor cells, making it possible to determine the size of brain tumors.
Unlike the amino acids mentioned above, threonine is of lesser importance: amino acids are not synthesized from it, and its content in tissues is low. The main value of threonine is its inclusion in proteins. This amino acid has no specific functions.
Asparagine, lysine, glutamine
Asparagine is a common nonessential amino acid present as the sweet-tasting L-isomer and the bitter-tasting D-isomer. Body proteins are formed from asparagine, and oxaloacetate is synthesized through gluconeogenesis. This substance can be oxidized in the tricarboxylic acid cycle and provide energy. This means that in addition to the structural function, asparagine also performs an energetic one.
Lysine, which cannot be synthesized in the human body, is an amino acid with alkaline properties. Immune proteins, enzymes and hormones are mainly synthesized from it. Moreover, lysine is an amino acid that independently exhibits antiviral agents against the herpes virus. However, the substance is not used as a drug.
The amino acid glutamine is present in the blood in concentrations much higher than other amino acids. It plays a major role in the biochemical mechanisms of nitrogen metabolism and the excretion of metabolites, participates in the synthesis of nucleic acids, enzymes, hormones, and is capable of strengthening the immune system, although it is not used as a drug. But glutamine is widely used among athletes, as it helps to recover after training and removes nitrogen and butyrate metabolites from the blood and muscles. This mechanism for accelerating the athlete’s recovery is not considered artificial and is not rightly recognized as doping. Moreover, there are no laboratory methods for convicting athletes of such doping. Glutamine is also present in significant quantities in food.
How to get amino acids?
The role of AK in people's lives is of enormous importance at any period of life. To restore this complex and necessary balance during a normal rhythm of life, it is enough to consume foods rich in amino acids. However, this is not enough for professional athletes, people seeking to build muscle mass, and for those whose profession or lifestyle involves increased stress of an extreme nature. In such cases, it is better to use special organic complexes with a high content of amino acids.
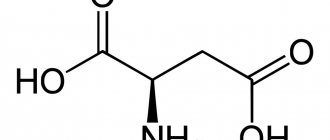
Aspartic and glutamic acid
Aspartic and glutamic amino acids are extremely valuable to the human body due to their neurotransmitter-activating properties. They speed up the transfer of information between neurons, ensuring the maintenance of the functioning of brain structures lying below the cortex. In such structures, reliability and constancy are important, because these centers regulate breathing and blood circulation. Therefore, there is a huge amount of aspartic and glutamic amino acids in the blood. The spatial structural formula of amino acids is shown in the figure below.
Aspartic acid is involved in the synthesis of urea, eliminating ammonia from the brain. It is an important substance for maintaining a high rate of reproduction and renewal of blood cells. Of course, in leukemia this mechanism is harmful, and therefore, to achieve remission, enzyme preparations that destroy the aspartic amino acid are used.
One fourth of all amino acids in the body is glutamic acid. This is a neurotransmitter of postsynaptic receptors, necessary for synaptic transmission of impulses between neuron processes. However, glutamic acid is also characterized by an extrasynaptic pathway of information transmission - volumetric neurotransmission. This method underlies memory and represents a neurophysiological mystery, because it has not yet been clarified which receptors determine the amount of glutamate outside the cell and outside the synapses. However, it is the amount of substance outside the synapse that is thought to be important for bulk neurotransmission.
Purpose of essential amino acids
Proteins and NAC are of great importance for the proper functioning of all systems of the human body. Protein that comes from outside is the most complete when it has a balanced composition. The need for NAC increases sharply with intense sports and a high risk of injury. It is impossible to build muscle mass if there is a lack of AA in the body. To quickly recover from physical and stressful activities, burn fat and maintain excellent shape, it is necessary to consume foods rich in NAC for their correct balance in the complex system of human functioning.
Chemical structure
All non-standard and 20 standard amino acids have a common structure plan. It includes a cyclic or aliphatic hydrocarbon chain with or without the presence of radicals, an amino group at the alpha carbon atom and a carboxyl group. The hydrocarbon chain can be anything, in order for a substance to have the reactivity of amino acids, the location of the main radicals is important.
The amino group and carboxyl group must be attached to the first carbon atom of the chain. According to the nomenclature accepted in biochemistry, it is called an alpha atom. This is important for the formation of a peptide group, the most important chemical bond that makes proteins exist. From the point of view of biological chemistry, life is the mode of existence of protein molecules. The main importance of amino acids is the formation of peptide bonds. The general structural formula of amino acids is presented in the article.
Physical properties
Despite the similar structure of the hydrocarbon chain, amino acids have significantly different physical properties from carboxylic acids. At room temperature they are hydrophilic crystalline substances and are highly soluble in water. In an organic solvent, due to dissociation at the carboxyl group and the removal of a proton, amino acids dissolve poorly, forming mixtures of substances, but not true solutions. Many amino acids taste sweet, while carboxylic acids taste sour.
These physical properties are due to the presence of two functional chemical groups, due to which the substance behaves in water like a dissolved salt. Under the influence of water molecules, a proton is removed from the carboxyl group, the acceptor of which is the amino group. Due to the shift in the electron density of the molecule and the absence of freely moving protons, pH (an indicator of acidity) the solution remains quite stable when acids or alkalis with high dissociation constants are added. This means that amino acids are able to form weak buffer systems, maintaining homeostasis in the body.
It is important that the charge modulus of a dissociated amino acid molecule is zero, since the proton removed from the hydroxyl group is accepted by the nitrogen atom. However, a positive charge is formed on the nitrogen in the solution, and a negative charge is formed on the carboxyl group. The ability to dissociate directly depends on acidity, and therefore there is an isoelectric point for amino acid solutions. This is the pH (a measure of acidity) at which the largest number of molecules have zero charge. In this state, they are motionless in the electric field and do not conduct current.
Lecture No. 16. Amino acids. Peptides
- Shares
Lecture No. 1765
AMINO ACIDS. PEPTIDES
Plan
- Receipt methods.
- Chemical properties.
- Amino acids that make up proteins.
- Peptides
Lecture No. 16
AMINO ACIDS. PEPTIDES
Plan
- Receipt methods.
- Chemical properties.
- Amino acids that make up proteins.
- Peptides
Amino acids are heterofunctional compounds containing carboxyl and amino groups. Based on the relative arrangement of functional groups, a -, b -, g -, etc. are distinguished. amino acids. Amino acids containing an amino group at the end of the chain are called w-amino acids.
1. Methods of obtaining
!) Ammonolysis of halogenated acids.
The method is used for the synthesis of a-amino acids from available a-halogenated acids.
2) Stecker-Zelinsky method
Includes the stages of aminonitrile formation through the interaction of aldehyde with HCN and NH3, followed by its hydrolysis into an amino acid. A mixture of NaCN and NH4Cl is used as a reagent.
The method is applicable for the synthesis of only a-amino acids.
3) Reductive amination of oxoacids
4) Addition of ammonia to a,b-unsaturated carboxylic acids.
The method is applicable for the synthesis of b-amino acids.
5) From oximes of cyclic ketones by Beckmann rearrangement.
The method is used for the synthesis of w-amino acids.
2. Chemical properties
Amino acids give reactions characteristic of carboxyl and amino groups, and, in addition, exhibit specific properties that are determined by the presence of two functional groups and their mutual arrangement.
2.1. Acid-base properties
Amino acids contain acidic and basic centers and are amphoteric compounds. In the crystalline state, they exist as internal salts (bipolar ions), which are formed as a result of intramolecular proton transfer from a weaker basic center (COO—) to a stronger basic center (NH2).
The ionic structure of amino acids is confirmed by their physical properties. Amino acids are non-volatile crystalline substances with high melting points. They are insoluble in non-polar organic solvents and soluble in water. Their molecules have large dipole moments.
The form of existence of amino acids in aqueous solutions depends on pH. In acidic solutions, amino acids add a proton and exist primarily as cations. In an alkaline environment, a bipolar ion gives up a proton and becomes an anion.
At a certain pH value, strictly defined for each amino acid, it exists predominantly in the form of a bipolar ion. This pH value is called the isoelectric point
(
pI ).
At the isoelectric point, an amino acid has no charge and is least soluble in water. The cationic form of the amino acid contains two acidic centers (COOH and NH3+) and is characterized by two dissociation constants pKa1 and pKa2. The pI value is determined by the equation:
2.2. Reactions on the amino group
Deamination
Amino acids contain a primary amino group and, like primary amines, react with nitrous acid to release nitrogen. In this case, the amino group is replaced by a hydroxyl group.
RCH(NH2)COOH + HNO2 ® RCH(OH)COOH + N2 + H2O
The reaction is used to quantify amino acids based on the volume of nitrogen released (Van-Slyke method).
Alkylation and arylation
When amino acids react with an excess of alkyl halide, exhaustive alkylation of the amino group occurs and internal salts are formed.
Amino acids are arylated with 2,4-dinitrofluorobenzene (DNFB) in an alkaline medium. The reaction proceeds as a nucleophilic substitution on the activated aromatic ring.
The reaction is used to determine the amino acid sequence of peptides.
Acylation
Amino acids react with anhydrides and acid chlorides to form N-acyl derivatives.
The reaction is used to protect the amino group in peptide synthesis. Such protection should be easy to remove, and amides are known to hydrolyze under harsh conditions. During the development of methods for the synthesis of peptides, protecting groups were found that are easily removed by hydrolysis or hydrogenolysis.
Carbobenzoxy protection:
tert-Butoxycarbonyl protection (BOK protection).
The ease of deprotection is due to the stability of benzyl and tert
-butyl cations, which are formed as intermediates.
2.3. Reactions on the carboxyl group
Decarboxylation
During dry distillation in the presence of barium hydroxide, amino acids are decarboxylated to form amines.
Esterification
Amino acids react with alcohols in the presence of gaseous HCl as a catalyst to form esters.
Unlike amino acids themselves, their esters are highly volatile compounds and can be separated by distillation or gas-liquid chromatography, which is used to analyze and separate mixtures of amino acids obtained from the hydrolysis of proteins.
Preparation of acid halides and anhydrides
When phosphorus or sulfur halides act on amino acids protected by the amino group, acid chlorides are formed.
The reaction is used to activate the carboxyl group through nucleophilic substitution. More often, mixed anhydrides are prepared for this purpose, which are more selective acylating reagents.
The reaction is used to activate the amino group in the synthesis of peptides.
2.4. Specific reactions of amino acids
Reactions with the simultaneous participation of carboxyl and amino groups occur, as a rule, with the formation of products containing thermodynamically stable 5- and 6-membered heterocycles.
Complexation
a-Amino acids form strong chelate complexes with transition metal ions (Cu, Ni, Co, Cr, etc.).
Relation of amino acids to heat
Transformations of amino acids upon heating depend on the relative position of the carboxyl and amino groups and are determined by the possibility of the formation of thermodynamically stable 5-6-membered rings
a-Amino acids undergo intermolecular self-acylation. In this case, cyclic amides are formed - diketopiperazines.
b-Amino acids become a,b-unsaturated acids when heated.
g- and d-amino acids undergo intramolecular acylation with the formation of cyclic amides - lactams
.
Ninhydrin reaction
When a-amino acids interact with triketone - ninhydrin, simultaneous oxidative deamination and decarboxylation occur with the formation of aldehyde and a colored condensation product.
The reaction is used for quantitative analysis of amino acids by photometry.
- a-Amino acids that make up proteins
3.1.
Structure and classification Natural amino acids correspond to the general formula RCH(NH2)COOH and differ in the structure of the radical R. The formulas and trivial names of the most important amino acids are given in the table. For the biological functioning of amino acids in proteins, the polarity of the R radical is decisive. On this basis, amino acids are divided into the following main groups (see table).
Table. The most important
a-amino acids RCH(NH2)COOH
| Formula | Name | Designation | pI |
| Amino acids containing a non-polar radical R | |||
| Glycine | Gly | 5,97 | |
| Alanin | Ala | 6,0 | |
| Valin | Val | 5,96 | |
| Leucine | Leu | 5,98 | |
| Isoleucine | Ile | 6,02 | |
| Phenylalanine | Phe | 5,48 | |
| Tryptophan | Trp | 5,89 | |
| Proline | Pro | 6,30 | |
| Methionine | Met | 5,74 | |
| Cystine | (Cys)2 | 5,0 | |
| Amino acids containing a polar nonionic radical R | |||
| Serin | Ser | 5,68 | |
| Threonine | Thr | 5,60 | |
| Hydroxyproline | Hyp | 5,8 | |
| Aspargine | Asn | 5,41 | |
| Glutamine | Gln | 5,65 | |
| Amino acids containing a polar, positively charged radical R | |||
| Lysine | Lys | 9,74 | |
| 5-Hydroxylysine | 9,15 | ||
| Arginine | Arg | 10,76 | |
| Histidine | His | 7,59 | |
| Amino acids containing a polar negatively charged radical R | |||
| Aspartic acid | Asp | 2,77 | |
| Glutamic acid | Gly | 3,22 | |
| Tyrosine | Tyr | 5,66 | |
| Cysteine | Cys | 5,07 | |
Amino acids containing a non-polar radical R. Such groups
are located inside the protein molecule and cause hydrophobic interactions.
Amino acids containing a polar nonionic radical R.
Amino acids of this type have polar groups in the side radical that are not capable of ionization in an aqueous environment (alcoholic hydroxyl, amide group). Such groups can be located both inside and on the surface of the protein molecule. They participate in the formation of hydrogen bonds with other polar groups.
Amino acids containing radical R, capable of ionization in an aqueous environment to form positively or negatively charged groups.
Such amino acids contain in the side radical an additional basic or acidic center, which in an aqueous solution can, respectively, add or donate a proton.
In proteins, the ionogenic groups of these amino acids are located, as a rule, on the surface of the molecule and cause electrostatic interactions.
3.2. Stereoisomerism.
All natural a-amino acids (except glycine) are chiral compounds. Based on the configuration of the chiral center at position 2, amino acids are assigned to the D- or L-series.
Natural amino acids belong to the L-series.
Most amino acids contain one chiral center and have two stereoisomers. The amino acids isoleucine, threonine, hydroxyproline, 5-hydroxylysine and cystine contain two chiral centers and have (except cystine) 4 stereoisomers, of which only one is found in proteins.
Thus, of the 4 stereoisomers of threonine, only (2S,3R)-2-amino-3-hydroxybutanoic acid occurs in nature.
The use of only one type of stereoisomer to build proteins is important for the formation of their spatial structure and ensuring biological activity.
a-Amino acids obtained synthetically are racemic mixtures that must be separated. The most preferred is the enzymatic separation method using acylase enzymes capable of hydrolyzing N-acetyl derivatives of La amino acids only. Enzymatic digestion is carried out according to the following scheme.
First, the racemic amino acid is acylated with acetic anhydride:
The racemic mixture of acetyl derivatives is then subjected to enzymatic treatment. In this case, only the acetyl derivative of the L-amino acid is hydrolyzed:
The mixture obtained after the enzymatic process is easily separated, since the free L-amino acid dissolves in both acids and alkalis, and the acylated one dissolves only in alkalis.
3.3. Acid-base properties.
Based on their acid-base properties, amino acids are divided into three groups.
Neutral amino acids
The R radical does not contain additional acidic or basic sites capable of ionization in an aqueous environment. In an acidic environment they exist as a singly charged cation and are dibasic acids according to Brønsted. As can be seen from the example of alanine, the isoelectric point of neutral amino acids is not equal to 7, but lies in the range of 5.5 – 6.3.
pI=1/2(2.34+9.69)=6.01
Essential amino acids
contain an additional basic center in the R radical. These include lysine, histidine and arginine. In an acidic environment they exist as dications and are tribasic acids. The isoelectric point of basic amino acids, as seen in the example of lysine, lies in the pH region above 7.
pI= 1/2(9.0+10.05)=9.74
Acidic amino acids
contain an additional acid center in the R radical. These include aspartic and glutamic acids. In an acidic environment they exist as a cation and are tribasic acids. The isoelectric point of these amino acids lies in the pH region well below 7.
pI= 1/2(2.09+3.86)=2.77
Tyrosine and cysteine contain weak acidic sites in their side radicals that are capable of ionization at high pH values.
It is important that at physiological pH (~7) not a single amino acid is at the isoelectric point. In the body, all amino acids are ionized, which ensures their good solubility in water.
The difference in acid-base properties is used to separate amino acids by electrophoresis and ion exchange chromatography. At a given pH value, different amino acids can have electrical charges of different magnitude and sign. For example, at pH6, lysine has a charge of +1 and moves to the cathode, aspartic acid has a charge of –1 and moves to the anode, and alanine is at the isoelectric point and does not move in the electric field. Thus at pH6 they can be separated by electrophoresis.
To separate amino acids by ion exchange chromatography, cation exchange resins (sulfonated polystyrene) are used. The process is carried out in an acidic environment when the amino acids are in cationic form.
The rate at which amino acids move through a chromatographic column depends on the strength of their electrostatic and hydrophobic interactions with the resin. Basic amino acids that have the greatest positive charge bind most strongly to the resin, while acidic amino acids bind least strongly to the resin. Amino acids with nonpolar side radicals, especially aromatic ones, have the greatest hydrophobic binding to the resin. Thus, the order of elution of amino acids is as follows. Acidic amino acids (Asp and Glu) elute more easily than others, followed by amino acids containing polar nonionic groups (Ser, Thr, Asn, Gln), then amino acids with non-polar side radicals (Phe, Trp, Ile, etc.) are eluted from the column. and lastly the basic amino acids (His, Lys, Arg) are eluted.
- 3.4. Amino acid reactions in vivo
Reductive amination
– a method for the synthesis of a-amino acids from a-oxoacids with the participation of the coenzyme NADH as a reducing reagent.
Trasamination
–
the main pathway for the biosynthesis of amino acids.
During transamination, an interchange of two functional groups - amine and carbonyl - occurs between the amino acid and the keto acid. In this case, amino acid 1, necessary for the body, is synthesized from amino acid 2, which is available in excess quantities in cells. The reaction is carried out with the participation of the enzymes transaminases and the coenzyme pyridoxal phosphate. Containing an aldehyde group, pyridoxal phosphate serves as a carrier of an amino group in the form of a Schiff base.
Decarboxylation
Amino acids are decarboxylated by decarboxylase enzymes with the participation of the coenzyme pyridoxal phosphate. In this case, biogenic amines are formed.
Biogenic amines have pronounced biological activity. The most important of them are - colamine (precursor in the synthesis of choline and the neurotransmitter acetylcholine), histamine (provides allergic reactions of the body), g-aminobutyric acid (neurotransmitter), adrenaline (adrenal hormone, neurotransmitter)
Deamination
Non-oxidative deamination occurs by the elimination of ammonia under the action of enzymes with the formation of a,b-unsaturated acids.
Oxidative deamination occurs with the participation of oxidase enzymes and the coenzyme NAD+, which acts as an oxidizing agent. As a result, ammonia is released and the corresponding keto acid is formed.
With the help of deamination reactions, excess amino acids in the body are reduced.
4. Peptides
Peptides are polyamides built from a-amino acids. Based on the number of amino acid residues in a peptide molecule, dipeptides, tripeptides, and tetrapeptides
etc.
Peptides containing up to 10 amino acid residues are called oligopeptides
, more than 10 amino acid residues are
polypeptides
.
Natural polypeptides containing more than 100 amino acid residues are called proteins
.
4.1. Peptide structure
Formally, peptides can be considered as polycondensation products of amino acids.
Amino acid residues in a peptide are linked by amide ( peptide)
) connections.
One end of the chain, at which there is an amino acid with a free amino group, is called N-terminus
.
The other end, which contains an amino acid with a free carboxyl group, is called the C-terminus
. Peptides are usually written and named starting from the N-terminus.
The name of the peptide is based on the trivial names of the amino acid residues included in its composition, which are listed starting from the N-terminus. At the same time, in the names of all amino acids, with the exception of the C-terminal suffix “in”, the suffix “il” is replaced. For the abbreviated designation of peptides, three-letter designations of the amino acids included in its composition are used.
The peptide is characterized by its amino acid composition
and
amino acid sequence
.
The amino acid composition of the peptide can be determined by complete hydrolysis of the peptide (cleavage to amino acids), followed by qualitative and quantitative analysis of the resulting amino acids using ion exchange chromatography or GLC analysis of amino acid esters. Complete hydrolysis of peptides is carried out in an acidic environment by boiling them with 6N. HCl.
Several peptides have the same amino acid composition. So, from 2 different amino acids 2 dipeptides can be built, from three different amino acids - 6 tripeptides, from n different amino acids n! peptides of the same composition. For example, the following 6 tripeptides correspond to the composition Gly:Ala:Val=1:1:1.
Gly-Ala-Val Gly-Val-Ala Val-Gly-Ala Val-Ala-Gly Ala-Gly-Val Ala-Val-Glu
Thus, to fully characterize a peptide, it is necessary to know its amino acid composition and amino acid sequence.
4.2. Amino acid sequence determination
To determine the amino acid sequence, a combination of two methods is used: determination of terminal amino acids and partial hydrolysis.
Determination of N-terminal amino acids.
Segner method. The peptide is treated with 2,4-dinitrophrobenzene (DNPB) and then completely hydrolyzed. The DNP derivative of the N-terminal amino acid is isolated and identified from the hydrolyzate.
The Edman method consists of reacting the N-terminal amino acid with phenylisothiocyanate in an alkaline medium. Upon further treatment with a weak acid without heating, the “labeled” terminal amino acid is separated from the chain in the form of a phenylhydantoin (PH) derivative.
The advantage of this method is that when the N-terminal amino acid is removed, the peptide is not destroyed and the removal operation can be repeated. The Edman method is used in an automatic device - a sequencer, with which it is possible to carry out 40 - 50 stages of elimination, identifying the PTG derivatives obtained at each stage using gas-liquid chromatography.
Partial hydrolysis of polypeptides
During partial hydrolysis, the peptides are broken down to form shorter chains. Partial hydrolysis is carried out using enzymes that hydrolyze peptide bonds selectively, for example, only from the N-terminus ( aminopeptidases
) or only from the C-terminus (
carboxypeptidases
). There are enzymes that break down peptide bonds only between certain amino acids. By changing the hydrolysis conditions, it is possible to break the peptide into various fragments that overlap in their constituent amino acid residues. Analysis of partial hydrolysis products makes it possible to reconstruct the structure of the original peptide. Let's consider the simplest example of establishing the structure of a tripeptide. Partial hydrolysis in two different directions of a tripeptide of unknown structure gives the products shown in the diagram.
The only tripeptide whose structure does not contradict the products of partial hydrolysis is Gly-Ala-Phe.
Establishing the amino acid sequence of peptides containing several dozen amino acid residues is a more complex task that requires a combination of various methods.
4.3. Peptide synthesis
Synthesis of a peptide with a given amino acid sequence is an extremely difficult task. In the simplest case of dipeptide synthesis from 2 different amino acids, the formation of 4 different products is possible.
Currently, a strategy for peptide synthesis has been developed based on the use of activation
and
protection
of functional groups at appropriate stages of the synthesis. The process of dipeptide synthesis includes the following stages:
- protection of the amino group of the N-terminal amino acid;
- activation of the carboxyl group of the N-terminal amino acid;
- condensation of modified amino acids
- deprotection
Thus, by sequentially adding amino acids, the polypeptide chain is increased step by step. This synthesis is very time-consuming, labor-intensive and gives a low yield of the final product. The main losses are associated with the need to isolate and purify products at each stage.
The currently used solid-phase peptide synthesis
. At the first stage, the amino-protected C-terminal amino acid is fixed on a solid polymer carrier (polystyrene modified by the introduction of –CH2Cl groups). After deprotection, the amino group of the amino acid attached to the carrier is acylated with another amino acid that contains an activated carboxyl and protected amino group. After deprotection, the next acylation step is carried out. Washing of the product from impurities is carried out directly on the carrier and only after completion of synthesis the polypeptide is removed from the carrier by the action of hydrobromic acid. Solid-phase synthesis is automated and carried out using devices - automatic synthesizers.
;
The method of solid-phase synthesis has been used to obtain a large number of peptides containing 50 or more amino acid residues, including insulin (51 amino acid residues) and ribonuclease (124 amino acid residues).