The processes of muscle work represent a multi-level complex of physiological and biochemical functions that are vital for the full functioning of the human body. Externally, similar processes can be observed in the examples of voluntary movements when walking, running, changing facial expressions, etc. However, they cover a much wider range of functions, which also include the work of the respiratory apparatus, digestive organs and excretory system. In each case, the mechanism of muscle contraction is supported by the work of millions of cells, which involve chemical elements and physical fibers.
Muscles
Muscles are grouped into groups that have the same mechanism of muscle contraction. On the same basis, they are divided into 3 types:
- striated muscles of the body;
- striated muscles of the atria and cardiac ventricles;
- smooth muscles of organs, blood vessels and skin.
The striated muscles are part of the musculoskeletal system, being part of it, since in addition to them it also includes tendons, ligaments, and bones. When the mechanism of muscle contraction is implemented, the following tasks and functions are performed:
- the body moves;
- body parts move relative to each other;
- the body is supported in space;
- heat is generated;
- the cortex is activated by afferentation from receptive muscle fields.
Smooth muscle consists of:
- the motor apparatus of the internal organs, which includes the bronchial tree, lungs and digestive tube;
- lymphatic and circulatory systems;
- genitourinary system.
Features of excitation at the neuromuscular synapse
Unilateral conduction of excitation - only in the direction from the presynaptic terminal to the postsynaptic membrane.
Summation of excitation of neighboring postsynaptic membranes.
Synaptic delay - the slowdown in the conduction of an impulse from a neuron to a muscle is 0.5-1 ms . This time is spent on the secretion of the transmitter, its diffusion to the postsynaptic membrane, interaction with the receptor, formation of the EPP, and their summation.
Low lability - it is 100-150 pulses/s for a signal, which is 5-6 times lower than the lability of the nerve fiber .
Sensitivity to the action of drugs, poisons, biologically active substances that act as a mediator.
Fatigue of chemical synapses - is expressed in deterioration of conductivity up to blockade in the synapse during long-term functioning of the synapse. The main cause of fatigue is the depletion of transmitter reserves in the presynaptic terminal.
Laws for the conduction of excitation through nerves:
- Law of functional integrity of the nerve.
- Law of isolated conduction of excitation.
- The law of two-way conduction of excitation.
Depending on the speed of excitation, nerve fibers are divided into 3 groups: A, B, C. In group A there are 4 subgroups: alpha, beta, gamma and sigma.
Physiological properties
Like all vertebrates, the human body has three most important properties of skeletal muscle fibers:
- contractility - contraction and change in tension during excitation;
- conductivity - movement of potential throughout the fiber;
- excitability is a response to a stimulus by changing the membrane potential and ionic permeability.
The muscles become excited and begin to contract from nerve impulses coming from the centers. But in artificial conditions, electrical stimulation is used. The muscle can then be stimulated directly (direct stimulation) or through the nerve innervating the muscle (indirect stimulation).
Source of energy for reduction
As we already know, muscle contraction is the movement (opening and closing) of transverse bridges, and naturally, this process requires energy supply. However, the energy reserve in the muscles is very small, so it must be constantly “drawn” from somewhere and restored.
We invite you to familiarize yourself with Documents for divorce through the court in 2019: complete list
The main and main supplier of energy, thanks to which the body is supplied with energy, is the ATP molecule, which is broken down thanks to the heads of myosin bridges, thus generating energy for muscle contraction. However, a muscle cell contains a limited supply of ATP molecules, their quantity is enough for only 8 repetitions, the first 2 seconds:
- ATP H2O = ADP H3PO4 energy
The problem of limited energy supply for muscle contraction is easily solved by resynthesis or re-creation of ATP molecules.
ATP is a universal source of human energy.
Depending on the time the muscles are under load, as well as on the degree of intensity of performing a particular exercise, the following types of systems can be distinguished that provide energy to the muscles:
- Phosphogenic (ATP and KrP)
- Glycolytic (anaerobic and aerobic glycolysis)
- Oxidation (carbohydrates and lipids are broken down with active oxygen supply)
2 Pyruvate 4ATP 2GTP 2NADH 2H 4H2O → glucose 4ADP 2GDP 6Pi 2NAD
Each energy system is characterized by power and capacity.
Power is an indicator that limits the intensity of physical work performed and characterizes the maximum amount of energy that is released per unit of time, as well as the maximum amount of resynthesized ATP per unit of time.
In turn, the capacity of energy systems limits the duration of muscle work.
To contract a muscle, the energy of ATP hydrolysis is used, but the muscle cell has an extremely efficient system for regenerating the ATP supply, so that in a relaxed and working muscle the ATP content is approximately equal. The enzyme phosphocreatine kinase catalyzes the reaction between ADP and creatine phosphate, the products of which are ATP and creatine.
Creatine phosphate contains more stored energy than ATP. Thanks to this mechanism, during a burst of activity in the muscle cell, the content of creatine phosphate drops, but the amount of the universal energy source - ATP - does not change. Mechanisms for regenerating ATP reserves may vary depending on the partial pressure of oxygen in surrounding tissues (see Anaerobic Organisms).
Types of abbreviations
The mechanism of muscle contraction involves the conversion of chemical energy into mechanical work. This process can be measured in an experiment with a frog: its calf muscle is loaded with a small weight and then stimulated with light electrical impulses. A contraction in which the muscle becomes shorter is called isotonic. With isometric contraction, no shortening occurs. Tendons do not allow the muscle to shorten as it develops strength. Another auxotonic mechanism of muscle contraction involves conditions of intense loads, when the muscle is shortened minimally and strength is developed to the maximum.
Contraction phases
When the muscles are activated by an irritating impulse of suprathreshold force, a single contraction occurs, in which 3 phases can be distinguished:
- The period of latent type contraction already mentioned above, during which the fibers accumulate energy to perform subsequent actions. At this time, electromechanical coupling processes take place and the centers of the ligaments open. At this stage, the mechanism of muscle fiber contraction is prepared, which is activated after the propagation of the corresponding impulse.
- The shortening phase lasts 50 ms on average.
- The relaxation phase also lasts approximately 50 ms.
Structure and innervation of skeletal muscles
Striated skeletal muscles contain many fibers found in connective tissue and attached to tendons. In some muscles, the fibers are located parallel to the long axis, while in others they have an oblique appearance, attaching to the central tendon cord and to the pennate type.
The main feature of the fiber is the sarcoplasm, a mass of thin filaments - myofibrils. They include light and dark areas, alternating with each other, and the neighboring striated fibers are at the same level - at the cross section. This results in transverse striation throughout the entire muscle fiber.
The sarcomere is a complex of a dark and two light disks, and is delimited by Z-shaped lines. Sarcomeres are the contractile apparatus of muscles. It turns out that the contractile muscle fiber consists of:
- contractile apparatus (myofibril system);
- trophic apparatus with mitochondria, Golgi complex and weak endoplasmic reticulum;
- membrane apparatus;
- supporting apparatus;
- nervous apparatus.
Muscle fiber is divided into 5 parts with their own structures and functions and is an integral part of muscle tissue.
Sarcomeres and filaments
Sarcomeres are segments of fibers that are separated by so-called Z-plates containing beta-actinin. Actin filaments extend from each plate, and the spaces are filled with thick myosin analogues. Actin elements, in turn, look like strings of beads twisted into a double helix. In this structure, each bead is an actin molecule, and in the areas with indentations in the helix there are troponin molecules. Each of these structural units forms a mechanism for contraction and relaxation of muscle fibers by communicating with each other. The cell membrane plays a key role in the excitation of fibers. It contains transverse invagination tubes that activate the function of the sarcoplasmic reticulum - this will be an exciting effect for muscle tissue.
Innervation
This process in striated muscle fibers is realized through nerve fibers, namely the axons of motor neurons in the spinal cord and brain stem. One motor neuron innervates several muscle fibers. The complex with a motor neuron and innervated muscle fibers is called a neuromotor unit (NME), or motor unit (MU). The average number of fibers that one motor neuron innervates characterizes the size of the muscle MU, and the inverse value is called innervation density. The latter is large in those muscles where movements are small and “subtle” (eyes, fingers, tongue). On the contrary, its small value will be in muscles with “rough” movements (for example, the torso).
Innervation can be single or multiple. In the first case, it is realized by compact motor endings. This is usually characteristic of large motor neurons. Muscle fibers (called physical or fast muscle fibers in this case) generate action potentials (APs) that are propagated to them.
Multiple innervations occur, for example, in the external eye muscles. No action potential is generated here, since there are no electrically excitable sodium channels in the membrane. In them, depolarization spreads throughout the fiber from the synaptic endings. This is necessary in order to activate the mechanism of muscle contraction. The process here does not happen as quickly as in the first case. That's why it's called slow.
Human muscular system
The human muscular system allows you to coordinate the movements of the body, keep it in balance, breathe, as well as transport food and blood within the body ; in addition, it protects the insides from damage, and also acts as a converter of chemical energy into mechanical and thermal.
There are only three types of muscles in the human body:
- skeletal
- smooth
- heart muscle
Human muscular system (A - cardiac muscle, B - skeletal muscle, C - smooth muscle)
Skeletal muscles
Human skeletal muscle, also known as striated muscle , is attached to the bones and consists of fibers, which in turn consist of muscle cells. Each muscle cell has two nuclei , which are responsible for division and repair. so-called myofibrils (threads), which are contained in muscle cells . The number of myofibrils in a muscle cell can reach up to several thousand. Thus, muscle cells form tissue , which in turn forms muscle.
Our skeletal muscles contain fibers, nerve endings and blood vessels. Muscle contraction occurs with the help of nerve impulses that come from the spinal cord to muscle tissue, that is, the transmission of the nerve impulse occurs along the path - brain → spinal cord → the muscles we need. Now we understand why spinal cord injury is so dangerous.
A person regulates the intensity of muscle contraction using the strength of the impulse supplied along the nerve endings.
Human skeletal muscles
Smooth muscle
Smooth muscle performs involuntary contractions, consists of spindle cells, being one of the most important components of the muscular hollow organs, as well as an integral part of the blood and lymphatic vessels, helps transport the contents of the hollow organs (transport food to the intestines), constricts the pupil, adjusts blood pressure, and other processes that occur involuntarily.
All smooth muscle contractions do not cause fatigue and are regulated by the autonomic system (autonomic nervous system, which is responsible for the functioning of internal organs).
You can train smooth muscles, for example, by increasing endurance , you improve the functioning of the cardiovascular system.
Smooth muscle
Heart muscle
The heart continuously contracts throughout life, providing movement, pumping blood, nutrients, and other vital substances through the vessels to the tissues of the body. Acting as a pump , the heart operates in a mode of continuous, rhythmic, single contractions.
The structure of myocardial resembles the structure of skeletal muscles, which also contain myofibrils consisting of actin and myosin, including the troponin-tropomyosin protein complex.
Picture of the heart, which shows the structure of the heart where you can see the myocardium
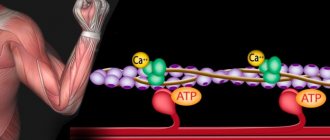
The mechanism of muscle contraction of the heart occurs for the same reasons as in striated muscles, thanks to Ca2+ (calcium) ions that are released from the sarcoplasmic reticulum (membrane organelle of muscle cells), only in this case it is less ordered (compared to skeletal muscles ).
Heart muscle and its structure
Myofibril structure
Studies of muscle fiber today are carried out on the basis of X-ray diffraction analysis, electron microscopy, and also histochemical methods.
It is calculated that each myofibril, whose diameter is 1 μm, contains approximately 2500 protofibrils, that is, elongated polymerized protein molecules (actin and myosin). Actin protofibrils are twice as thin as myosin protofibrils. At rest, these muscles are located in such a way that the actin filaments with their tips penetrate into the spaces between the myosin protofibrils.
The narrow light stripe in disk A is free of actin filaments. And the Z membrane holds them together.
Myosin filaments have transverse projections up to 20 nm long, the heads of which contain about 150 myosin molecules. They extend biopolarly, and each head connects a myosin filament to an actin filament. When there is a force on the actin centers on the myosin filaments, the actin filament moves closer to the center of the sarcomere. At the end, the myosin filaments reach the Z line. Then they occupy the entire sarcomere, and the actin filaments are located between them. In this case, the length of disk I is reduced, and at the end it disappears completely, at the same time the Z line becomes thicker.
Thus, according to the sliding filament theory, the reduction in muscle fiber length is explained. The theory, called the "gear wheel", was developed by Huxley and Hanson in the mid-twentieth century.
Optimum and pessimum frequency
The amplitude of contractions is determined by the frequency of impulses that irritate muscle fibers. In this system of interaction of signals and responses, an optimum and a pessimum of frequency can be distinguished. The first indicates the frequency, which at the moment of action will be superimposed on the phase of increased excitability. In this mode, the mechanism of muscle fiber contraction with a large amplitude can be activated. In turn, the pessimum determines a higher frequency, the impulse of which falls on the refractory phase. Accordingly, in this case the amplitude decreases.
Mechanism of muscle fiber contraction
The main thing in the theory is that it is not the filaments (myosin and actin) that are shortened. Their length remains unchanged even when the muscles are stretched. But bundles of thin threads, slipping, come out between thick threads, the degree of their overlap decreases, and thus contraction occurs.
The molecular mechanism of muscle contraction through the sliding of actin filaments is as follows. Myosin heads connect the protofibril to the actin fibril. When they tilt, sliding occurs, moving the actin filament towards the center of the sarcomere. Due to the bipolar organization of myosin molecules on both sides of the filaments, conditions are created for actin filaments to slide in different directions.
When muscles relax, the myosin head moves away from the actin filaments. Thanks to easy gliding, relaxed muscles resist stretching much less. Therefore, they passively lengthen.
Effect of stretching on contraction strength: resting stretch curve
rice.
2.9 The effect of pre-stretching on the force of muscle contraction. Pre-stretching increases muscle tension. The resulting curve describing the relationship between muscle length and the force of its contraction when exposed to active and passive stretching demonstrates a higher isometric tension than at rest. An important factor influencing the force of contraction is the amount of muscle stretch. Pulling the end of a muscle and pulling on the muscle fibers is called passive stretching. The muscle has elastic properties, however, unlike a steel spring, the dependence of tension on stretch is not linear, but forms an arcuate curve. As the stretch increases, the muscle tension also increases, but up to a certain maximum. The curve describing this relationship is called the resting stretch curve.
.
This physiological mechanism is explained by the elastic elements of the muscle - the elasticity of the sarcolemma and connective tissue, located parallel to the contractile muscle fibers.
Also, during stretching, the overlap of myofilaments on each other changes, but this does not affect the stretch curve, since at rest, cross-links between actin and myosin are not formed. Pre-stretching (passive stretching) is added to the force of isometric contractions (active contraction force).
Calcium ions
To better understand the process of fiber activation by calcium ions, it is convenient to consider the structure of the actin filament. Its length is about 1 micron, thickness - from 5 to 7 nm. This is a pair of twisted threads that resemble an actin monomer. Approximately every 40 nm there are spherical troponin molecules, and between the chains there are tropomyosin molecules.
When calcium ions are absent, that is, myofibrils relax, long tropomyosin molecules block the attachment of actin chains and myosin bridges. But when calcium ions are activated, tropomyosin molecules descend deeper and the areas open up.
Myosin bridges then attach to actin filaments, ATP is broken down, and muscle strength develops. This becomes possible due to the effect of calcium on troponin. In this case, the molecule of the latter is deformed, thereby pushing through tropomyosin.
When a muscle is relaxed, it contains more than 1 µmol of calcium per gram of wet weight. Calcium salts are isolated and stored in special storage facilities. Otherwise, the muscles would contract all the time.
Calcium is stored as follows. At different parts of the muscle cell membrane, there are tubes inside the fiber through which connection occurs with the environment outside the cells. This is a system of transverse tubes. And perpendicular to it there is a system of longitudinal ones, at the ends of which there are bubbles (terminal tanks), located in close proximity to the membranes of the transverse system. Together we get a triad. It is in the bubbles that calcium is stored.
This is how the PD spreads into the cell, and electromechanical coupling occurs. The excitation penetrates the fiber, passes into the longitudinal system, and releases calcium. This is how the muscle fiber contraction mechanism occurs.
Mechanics of muscle contractions[edit | edit code]
Source: “Sports Diagnostics”
Author:
Professor V.P. Guba, 2020
If a muscle is stimulated with a short electrical impulse, after a short latent period it contracts. This contraction is called a “single muscle contraction.” A single muscle contraction lasts about 10-50 ms, and it reaches maximum strength after 5-30 ms.
Each individual muscle fiber obeys the “all or nothing” law, i.e., when the force of stimulation is above a threshold level, a complete contraction occurs with the maximum force for a given fiber, and a stepwise increase in the force of contraction as the force of stimulation increases is impossible. Since the mixed muscle consists of many fibers with different levels of sensitivity to excitation, the contraction of the entire muscle can be stepwise depending on the strength of irritation, with strong irritations activating deeper muscle fibers.
Filament sliding mechanism[edit | edit code]
rice.
1. Scheme of the formation of cross-links - the molecular basis of sarcomere contraction Muscle shortening occurs due to the shortening of the sarcomeres that form it, which, in turn, are shortened due to the sliding of actin and myosin filaments relative to each other (and not the shortening of the proteins themselves). The theory of filament sliding was proposed by scientists Huxley and Hanson (Huxley, 1974; Fig. 1). (In 1954, two groups of researchers - H. Huxley with J. Hanson and A. Huxley with R. Niedergerke - formulated a theory explaining muscle contraction by the sliding of threads. Independently of each other, they found that the length of the A disk remained constant in relaxed and shortened sarcomere. This suggested that there are two sets of filaments - actin and myosin, and one fits into the spaces between the others, and as the length of the sarcomere changes, these filaments somehow slide over each other. This hypothesis is now accepted by almost everyone.)
Actin and myosin are two contractile proteins that are capable of entering into a chemical interaction, leading to a change in their relative position in the muscle cell. In this case, the myosin chain is attached to the actin filament using a number of special “heads”, each of which sits on a long springy “neck”. When coupling occurs between the myosin head and the actin filament, the conformation of the complex of these two proteins changes, the myosin chains move between the actin filaments, and the muscle as a whole shortens (contracts). However, in order for a chemical bond between the myosin head and the active filament to form, it is necessary to prepare this process, since in a calm (relaxed) state of the muscle, the active zones of the actin protein are occupied by another protein - tropochmyosin, which does not allow actin to interact with myosin. It is in order to remove the tropomyosin “cover” from the actin filament that a rapid pouring of calcium ions from the cisterns of the sarcoplasmic reticulum is required, which occurs as a result of the action potential passing through the muscle cell membrane. Calcium changes the conformation of the tropomyosin molecule, as a result of which the active zones of the actin molecule open for the attachment of myosin heads. This connection itself is carried out with the help of so-called hydrogen bridges, which very tightly bind two protein molecules - actin and myosin - and are able to remain in this bound form for a very long time.
To detach the myosin head from actin, it is necessary to expend adenosine triphosphate (ATP) energy, while myosin acts as an ATPase (an enzyme that breaks down ATP). The breakdown of ATP into adenosine diphosphate (ADP) and inorganic phosphate (P) releases energy, breaks the connection between actin and myosin, and returns the myosin head to its original position. Subsequently, cross-links can again form between actin and myosin.
In the absence of ATP, actin-myosin bonds are not destroyed. This is the cause of rigor mortis after death, because the production of ATP in the body stops - ATP prevents muscle rigidity.
Even during muscle contractions without visible shortening (isometric contractions, see above), the cross-linking cycle is activated, the muscle consumes ATP and produces heat. The myosin head is repeatedly attached to the same actin binding site, and the entire myofilament system remains motionless.
Attention
: The contractile muscle elements actin and myosin by themselves are not capable of shortening. Muscle shortening is a consequence of the mutual sliding of myofilaments relative to each other (filament sliding mechanism).
How does the formation of cross-links (hydrogen bridges) translate into movement? A single sarcomere shortens by approximately 5-10 nm per cycle, i.e. approximately 1% of its total length. By quickly repeating the cross-link cycle, shortening of 0.4 µm, or 20% of its length, is possible. Since each myofibril consists of many sarcomeres and cross-links are formed in all of them simultaneously (but not synchronously), their total work leads to a visible shortening of the entire muscle. The transmission of the force of this shortening occurs through the Z-lines of myofibrils, as well as the ends of the tendons attached to the bones, resulting in movement in the joints through which the muscles move in space parts of the body or advance the entire body.
ATP mechanism
After completion of the movement, the AFT molecule provides energy for the separation of myosin and actin involved in the reaction. The myosin heads separate and ATP is broken down into phosphate and ADP. At the end, a new ATP molecule is attached and the cycle resumes. This is the mechanism of muscle contraction and relaxation at the molecular level.
The activity of cross bridges will continue only as long as ATP hydrolysis occurs. If the enzyme is blocked, the bridges will not reattach.
With the onset of death of the organism, the level of ATP in the cells drops, and the bridges remain stably attached to the actin filament. This is how the stage of rigor mortis occurs.
Introduction
Diagram showing muscles in relaxed (above) and contracted (below) positions.
The basis of all types of muscle contraction is the interaction of actin and myosin. In skeletal muscle, myofibrils (about two-thirds of the dry weight of muscle) are responsible for contraction. Myofibrils are structures 1 - 2 microns thick, consisting of sarcomeres - structures about 2.5 microns long, consisting of actin and myosin (thin and thick) filaments and Z-disks connected to actin filaments.
ATP resynthesis
Resynthesis can be realized in two ways.
Through the enzymatic transfer of a phosphate group from creatine phosphate to ADP. Since the reserves of creatine phosphate in the cell are much larger than ATP, resynthesis occurs very quickly. At the same time, through the oxidation of pyruvic and lactic acids, resynthesis will occur slowly.
ATP and CP can disappear completely if resynthesis is disrupted by poisons. Then the calcium pump will stop working, as a result of which the muscle will irreversibly contract (that is, contracture will occur). Thus, the mechanism of muscle contraction will be disrupted.
Motor unit
It is now worth moving away from the in-depth structure of the muscle and considering the motor unit in the overall configuration of the skeletal muscle. This will be a collection of muscle fibers innervated by the processes of the motor neuron. The work of muscle tissue, regardless of the nature of the action, will be provided by the fibers included in one motor unit. That is, when a motor neuron is excited, the mechanism of muscle contraction is triggered within the same complex with the innervated processes. This division into motor neurons allows specific muscles to be targeted in a targeted manner without unnecessarily exciting adjacent motor units. In fact, the entire muscle group of one organism is divided into segments of motor neurons, which can unite to work on contraction or relaxation, or can act differently or alternately. The main thing is that they are independent of each other and work only with signals from their own group of fibers.
Physiology of the process
To summarize the above, we note that muscle fiber contraction consists of shortening the myofibrils in each of the sarcomeres. Myosin (thick) and actin (thin) filaments are connected at their ends in a relaxed state. But they begin sliding movements towards each other when the mechanism of muscle contraction is realized. Physiology (briefly) explains the process when, under the influence of myosin, the necessary energy is released to convert ATP into ADP. In this case, myosin activity will be realized only with a sufficient content of calcium ions accumulating in the sarcoplasmic reticulum.
Coupling of excitatory and contractile processes
In a quiet state, the fiber strands do not interact with each other through sliding, since the centers of the ligaments are closed by tropomyosin molecules. Excitation can only take place after electromechanical coupling. This process is also divided into several stages:
- When a neuromuscular synapse is activated, a so-called postsynaptic potential is formed on the myofibril membrane, accumulating energy for action.
- The exciting impulse, thanks to a system of tubes, spreads across the membrane and activates the reticulum. This process ultimately helps to remove barriers from the membrane channels through which troponin-binding ions are released.
- The protein troponin, in turn, opens the centers of actin bundles, after which the mechanism of muscle contraction becomes possible, but it also requires an appropriate impulse to begin.
- The use of the opened centers will begin at the moment when myosin heads join them according to the model described above.
The full cycle of these operations occurs on average in 15 ms. The period from the initial point of fiber excitation to complete contraction is called latent.
Major proteins of myofibrils
| Protein | Protein % | His pier. mass, kDa | Its function |
| Myosin | 44 | 510 | Main component of thick filaments. Forms bonds with actin. Moves along actin due to ATP hydrolysis. |
| Actin | 22 | 42 | Main component of thin filaments. During muscle contraction, myosin moves along it. |
| Titin | 9 | 2500 | A large flexible protein that forms a chain to bind myosin to the Z-disc. |
| Troponin | 5 | 78 | A complex of three proteins that regulates contraction when bound to Ca2 ions. |
| Tropomyosin | 5 | 64 | A rod-shaped protein associated with actin filaments that blocks myosin movement. |
| Nebulin | 3 | 600 | A long, inextensible protein associated with the Z-disk and running parallel to actin filaments. |
Roll and lock: turn and lock
According to the lever hypothesis, in muscle contraction there is only one moment of force generation - when the myosin tail (lever) turns. However, some data from radiography and tomography of muscles not only do not agree with this theory, but indicate that there is still some incomprehensible moment in muscle contraction that the lever hypothesis does not explain. Therefore, a group of researchers led by A.K. Tsaturyan proposed a theory of muscle contraction called “Roll and lock” (see: Michael A. Ferenczi et al., 2005. The “Roll and Lock” Mechanism of Force Generation in Muscle). According to this theory, myosin heads sit on actin even before ATP hydrolysis, and they sit not in a slender and organized manner, but at random. On the myosin head there is a long protruding domain - a “probe” - which “gropes” for a suitable (acidic and negatively charged) part of the actin filament and sticks to it - as necessary, at the first angle that comes along. However, as soon as ATP hydrolysis occurs, myosin changes its conformation, the heads rotate at the desired angle and firmly and clearly, like a key with a lock, adhere to the actin filament, and phosphate is released from the myosin pocket. And only after this the lever turns. In other words, the model turns out to be two-stage: at the first stage, the myosin head firmly and clearly clings to the actin and at the same time rotates slightly, and at the second, the lever turns, and the force that will then lead to muscle movement is generated at both of these stages.
In addition to X-ray structural and tomographic data, which are in very good agreement with the “Roll and lock” theory, there are several indirect, but very beautiful evidence of its correctness. For example, it is known that during muscle contraction, if the muscle does not change its length, only a little more than 40% of the myosin heads sit on actin, and the rest dangle unattached to anything. However, when a contracted muscle is forcibly stretched (for example, this happens when running, when a person lands on a tense muscle), the stiffness of the muscle increases sharply due to the fact that almost all the free myosin heads are sharply coupled to the actin filament. However, judging by the X-ray diffraction data, they do not interlock “tightly”, like a key with a lock, but simply haphazardly. This can be explained using the “Roll and lock” theory. ATP hydrolysis stops when a muscle is stretched (this is understandable: what is the point of wasting ATP if the work is not done by the muscle
, but
above the muscle
), and all myosin heads go into a state of “active actin search” - their protruding probe searches for the actin filament, feels for a suitable place on it and adheres to it - not firmly, not like a key with a lock, but haphazardly . However, in order to increase the stiffness of the muscle (and thereby protect the bones from fracture), this is enough.
Myosin sliding relative to actin
Myosin heads break down ATP and, due to the released energy, change conformation, sliding along actin filaments. The cycle can be divided into 4 stages:
- The free myosin head binds to ATP and hydrolyzes it to ADP and phosphate and remains associated with them. (A reversible process - the energy released as a result of hydrolysis is stored in the changed conformation of myosin).
- The heads bind weakly to the next actin subunit, the phosphate is released, and this leads to strong binding of the myosin head to the actin filament. This reaction is already irreversible.
- The head undergoes a conformational change that pulls the thick filament toward the Z-disc (or, equivalently, the free ends of the thin filaments toward each other).
- ADP is released, due to this the head is separated from the actin filament. A new ATP molecule attaches.
The cycle is then repeated until the concentration of Ca2+ ions decreases or the ATP supply is exhausted (as a result of cell death). The speed of myosin sliding along actin is ≈15 μm/sec. There are many (about 500) myosin molecules in the myosin filament and, therefore, during contraction, the cycle is repeated by hundreds of heads at once, which leads to fast and strong contraction. It should be noted that myosin behaves like an enzyme - an actin-dependent ATPase. Since each repetition of the cycle is associated with ATP hydrolysis, and therefore with a positive change in free energy, the process is unidirectional. Myosin moves along actin only towards the plus end.
Successive stages